Green Solvents: Definition, Examples and Types of Green Solvents
What are Green Solvents?
Green solvents, also known as environmentally friendly bio solvents which are derived from the processing of crops.
There are many types of green solvents like ionic liquids, supercritical fluids, water, and supercritical water. These green solvents are way much eco-friendly, less toxic, less hazardous than traditional volatile organic compounds (VOCs).
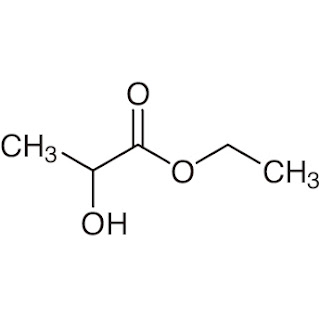
For example, Ethyl lactate
Ethyl lactate is a green solvent derived from processing corn. Ethyl lactate is the ester of lactic acid which is used as solvents in the paints and coating industry.
Types of Green Solvents
1. Supercritical Fluids
A compound that exists above its critical pressure (Pc) and above its critical temperature (Tc) is known as supercritical fluid or SCF. Their chemical and physical properties are between those of a gas and a liquid.
Phase diagram showing the supercritical fluid region
Supercritical liquids are the perfect replacement for organic solvents for industrial and lab processes due to their great solubility in many polymers.
Supercritical carbon dioxide (ScCO2) is a good example of supercritical liquids.
Supercritical Carbon dioxide (ScCO2)
Supercritical carbon dioxide (ScCO2) is known as a green solvent because it acts as a good solvent for many non-polar, few polar, and low molecular weight compounds.
Advantages of ScCO2 as solvent as compared to traditional organic solvents:
1. It is non-toxic.
2. It can be easily removed.
3. It is potentially recyclable.
4. It is non-flammable.
5. It has high gas solubility.
6. It has weak solvation.
7. It has high diffusion rates.
8. It is easy to handle.
9. It has good mass transfer.
10. It is readily available.
Disadvantages of ScCO2 as solvents as compared to traditional organic solvents:
1. Relatively high-pressure equipment.
2. Equipment can be capital intensive.
3. Relatively poor solvent.
4. Reactive with powerful nucleophiles.
5. Possible heat-transfer problems.
Application ScCO2 as a solvent
ScCO2 is widely used in polymerization. Polymerization of fluorine and silicon-containing monomers are done in ScCO2 as they are less soluble in organic solvents.
Free radical polymerization (using a free radical initiator such as AIBN) of acrylate monomers containing perfluoro-ponytails are also done in ScCO2.
2. Water
Water is a great solvent for most of the organic reactions. It is called green or universal solvent because it is non-toxic, easily available, and has a high specific heat capacity. There are many synthesis and reactions which are carried out in the water like Pericyclic reactions, reactions of radicals, and multicomponent reaction.
This is an example of a reduction reaction compared to high-temperature water.
Advantages of using water as a solvent for the organic reaction:
1. Water is non-toxic as compared to other organic solvents.
2. Water provides a great opportunity for replacing VOC's like Benzene, Toluene, etc.
3. Water is naturally occurring and easily available everywhere.
4. Water is non-flammable.
5. Water has a high specific heat capacity which means exothermic reactions can be more safely controlled.
Disadvantages of using water as a solvent for organic reaction
1. Water should be pure before using it in chemical reaction. So, distillation is costly and energy intensive.
2. Contaminated waste streams may be difficult to treat.
3. Water has high specific heat capacity which means its difficult to heat or cool rapidly.
3. Ionic liquids
An ionic liquid (IL) or ionic liquid is a molten salt in liquid state or we can say salts whose melting point is below some arbitary temperature like 100 C is called ionic liquid (IL).
Ionic liquid have poorly coordinated ions which make them liquid below 100 C or even at room temperature. At least one ion has a delocalized charge and one component is organic, which prevents the formation of a stable crystal lattice.
Properties of Ionic liquid
1. Ionic liquids are moderate to poor conductors of electricity, non-ionizing, highly viscous and exhibit low vapour pressure.
2. They have low combustibility, highly thermally stable and have wide liquid regions.
3. They have favourable solvating properties for many polar and non-polar compounds.
4. Ionic liquids can also serve as solvents for biocatalysis.
Applications of ionic liquid
1. By changing the cation/anion ratio, type and alkyl chain length properties like acidity/basically, melting temperature and viscosity can be changed to meet particular demands.
2. Due to stability of ionic liquids at temperatures above 300 C, they provide opportunity to carry out high-temperature reactions at low pressure.
3. Ionic liquids being immiscible with organic solvents or water, so they can be used in liquid-liquid extraction processes.
4. Fluorous biphasic solvent
Fluorous biphasic means fully fluorinated hydrocarbon solvents that are immiscible with organic solvents at ambient conditions. Reactants and catalysts should be soluble in the fluorous phase under reaction conditions but that products would readily separate into a distinct phase at ambient conditions.
The C-F bond in fluorous biphase solvents is very stable and provide it properties like non-toxicity, high level of inertness, highly thermal stability, low flammability and hydrophobicity.
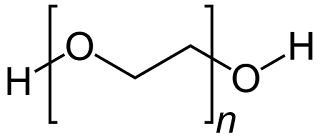
5. PEG (Polyethylene Glycol)
Polyethylene glycol (PEG) is a special type of green and inexpensive solvent used in various chemical synthesis. PEG has a low toxicity as compared to other organic solvents. Thus, it gives pure yield.
Advantages of using PEG as solvent
1. PEG is an eco-friendly solvent.
2. Various metal-catalyzed carbon-carbon bond formation reactions can conduct in PEG.
3. Reactions have excellent compatibility in both thermal and microwave irradiations.
4. Metal PEG system can be readily recover and reuse.