Malachite Green: Definition, Synthesis, Properties and Applications
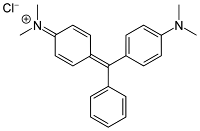
What is Malachite Green? Malachite green is a type of Triphenyl methane dye that is used as a dyestuff for materials like silk, leather, and paper. It is bright green and can be used as an antimicrobial agent in aquaculture. Synthesis of Malachite Green Malachite green can be prepared by reaction of Benzaldehyde with N, N-dimethyl aniline in the presence of H₂SO₄ to form a triphenylmethane derivative. The subsequent oxidation with PbO₂ followed by treatment with an excess of concentrated HCl yields a green dye known as malachite green. Properties of Malachite Green Malachite green is green, crystalline, and water-soluble. Leuco form of malachite green is electrically neutral and undergoes a photoionization reaction that yields the cationic form of malachite green. It is toxic, thus not advisable to consume. Applications of Malachite Green It is used as a dye to color materials like silk, leather, and paper. It is used as an antiseptic for bacterial infection. It is used to catch thi...

