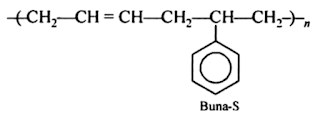
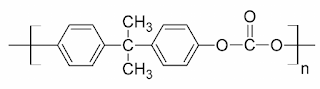
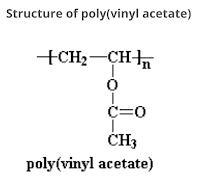
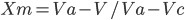Glass Transition Temperature (Tɡ): Definition, Significance and Factors Affecting
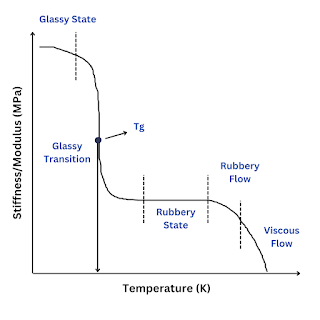
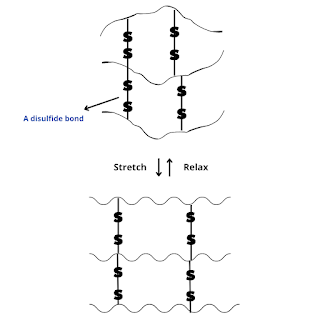
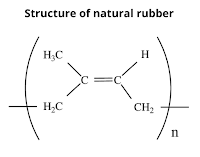
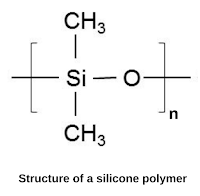
What is glass transition temperature? When plastic or rubber is cooled up to a certain temperature, it becomes so hard and brittle that it breaks into pieces on the application of stress. So, the temperature below which the polymer becomes hard, brittle, and glassy and above which it is softener and flexible is known as glass transition temperature (Tɡ). The glass transition is a property of only the amorphous region of a semi-crystalline solid whereas the crystalline portion remains crystalline during the glass transition. It is important to note that the transition does not occur suddenly at a unique temperature but rather over a range of temperatures. The temperature in the middle of the inclined region is taken as the Tɡ. Significance of glass transition temperature (Tg) Glass transition temperature (Tɡ) is used as a measure for evaluating the flexibility of a polymer and the type of response the polymeric material would exhibit to mechanical stress. Glass transition temperature (...
.png)