Types of Rubber, their Properties and Applications
1. Natural Rubber
Natural rubber is a natural polymer that is manufactured from latex which is a colloidal solution of rubber particles in water. Latex is obtained by making cuts in the bark of rubber trees like Hevea brasiliensis, found in tropical and semi-tropical countries such as southern.
India, Indonesia, Malaysia, Sri Lanka, South America, etc. The natural rubber has remarkable elasticity and undergoes long-range reversible extension even under a relatively small applied force.
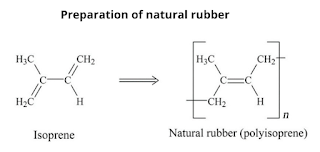
Preparation of natural rubber
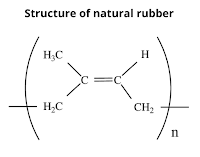
Natural rubber is a linear 1,4-addition polymer of isoprene.
Since each repeating unit in polyisoprene contains a double bond having cis-stereochemistry. That's why natural rubber is cis-polyisoprene.
Properties of Natural rubber
- Natural rubber has no polar groups and hence intermolecular forces of attraction are only weak van der Waals interactions.
- Cis-polyisoprene does not have a straight-chain but has a coiled structure. As a result, it can be stretched like a spring.
- Due to the coiled structure, natural rubber does not fit properly in the crystal lattice and hence is considered to be non-crystalline.
Applications of Natural rubber
- It has engineering applications like anti-vibration mounts, drive couplings, springs, bearings, rubber bands, etc.
- It is widely used in high-performance tires for race cars, buses, and aircraft due to its heat-resistant properties.
- It is used in hoses, automotive parts, foam mattresses, and battery boxes.
- Raw rubber is sometimes used for adhesives and as a part of shoe soles.
2. Synthetic Rubbers
Synthetic Rubber may be defined as any vulcanizable rubber-like polymer which is capable of getting stretched to twice its length. However, it returns to its original shape and size when the stretching force is taken out.
Most of these rubbers are derived from butadiene derivatives and contain carbon-carbon double bonds so that they can also be vulcanized. Thus, synthetic rubbers are either homopolymers of 1,3-butadiene or its derivatives or are copolymers of 1,3-butadiene or its derivatives with another unsaturated monomer.
Properties of Synthetic rubber
- Due to the trans orientation of double bonds, synthetic rubber has a highly regular zig-zag structure that cannot be stretched much. Hence, it is considered to be non-elastic.
- Due to the highly regular zig-zag structure, synthetic rubber fits closely in the crystal lattice and hence is considered to be crystalline.
Important Examples of Synthetic Rubbers
1. Chloroprene
Chloroprene is a monomer of neoprene. It is prepared by the addition of HCl to vinylacetylene. The solution takes place on the triple bond as per Markovnikov's rule.
Preparation of Chloroprene
Vinylacetylene needed for the purpose is prepared by dimerization of acetylene by passing it through an aqueous solution of ammonium chloride and cuprous chloride at 343 K.
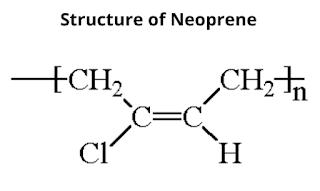
2. Neoprene
Neoprene (also known as polychloroprene), is a synthetic rubber that is made by polymerization of chloroprene. It is harder and stronger than natural rubber and resistant to water.
That's why it is used in the manufacture of hoses, gaskets, shoe heels, stoppers, etc.
Preparation of Neoprene
It is prepared by polymerization of chloroprene in which it polymerizes very readily (700 times faster than isoprene). The reaction occurs by 1,4-addition of one chloroprene molecule to the other where no specific catalyst is needed but the polymerization is slower in absence of oxygen.
Properties of Neoprene
- It has much more oil resistance than natural rubber. It is not oxidized by air.
- It has high tensile strength.
- It has acceptable chemical stability and maintains flexibility over a wide temperatures.
- It is resistant to sun, climate, and ozone determination.
Applications of Neoprene
- It is used as an insulator and for making conveyor belts and printing rollers.
- It is used in the manufacture of hoses, gaskets, shoe heels, stoppers, etc.
- During Covid-19, Neoprene is actively used to make face masks having 99.9 % filtration efficiency.
- It is a popular material in making protective clothing for aqua activities like scuba diving, swimming, etc.