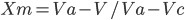Degree of Crystallinity: Definition, Factors Affecting and Determination Methods
What is Degree of Crystallinity?
The degree of crystallinity of the polymer is defined as the fraction of the sample which is crystalline. It can be either expressed in terms of the mass fraction or the volume fraction.
The degree of crystallinity by a volume fraction is given by
Where,
Xₘ = Degree of crystallinity by mass
V = Specific volume of the sample
Vₐ = Specific volume of fully amorphous polymer
Vc = Specific volume of fully crystalline polymer
Factors affecting the degree of crystallinity
1. Molecular weight
With the increase in molecular weight of the polymer, the degree of crystallinity increases due to a large number of entanglement of the chain which restricts the growth of a crystallite.
2. Symmetry of repeating unit
A symmetrical repeating unit structure like CH₂ makes it easier for the formation of crystallites. Thus, increasing the degree of crystallinity. That's why random copolymers do not crystallize because there is no regularity of the repeating unit.
3. Chain Branching
Branched chains are less likely to crystallize, thus decreasing the degree of crystallinity. For example, low-density polythene has short and long-chain branches. So, it cannot be obtained in a highly crystalline state whereas high-density polypropylene has an almost perfectly linear structure. Thus, it can be obtained in a highly crystalline state.
4. Cross-Linking
A polymer with high cross-linked density has a lower degree of crystallinity due to the presence of a dense array of cross-links that effectively eliminate crystallinity.
5. Secondary Bonding
Both inter and intra-molecular hydrogen bonding play important roles in the Degree of crystallinity of the polymer. With the increased chain-to-chain secondary bonding, there is an increase in the degree of crystallinity of the polymer.
Affect of crystallinity on the properties of Polymer
1. With the increased degree of crystallinity, strength, and stiffness of polymer increase but brittleness also increases.
2. With the increase of degree of crystallinity, solubility, and permeability of polymer decreases.
3. With the increase in the degree of crystallinity, the density and melting point of polymer decreases.
4. With the increase of the degree of crystallinity, the opacity of the polymer also increases.
Determination of degree of crystallinity
There are many ways from which we can calculate the degree of crystallinity including common methods like the density measurement method and Differential Scanning Calorimetry (DSC) method and other methods like X-ray analysis, IR spectroscopy, and NMR spectral analysis.
1. Density measurement method
The density measurement method is one of the most common methods for measuring the degree of crystallinity of semi-crystalline polymers. This technique relies on the differences between the densities of completely amorphous (Pa) and entirely crystalline domains (Pc) of the same polymer. It estimates the degree of crystallinity (Wc) from the densities of the real specimen (Ps).
Assuming that the volumes of the crystalline and amorphous domains are additive, we can write the formula to determine the degree of crystallinity. To get the percentage of the degree of crystallinity, we will simply multiply it by 100.
2. Differential scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) provides a quick method for determining the degree of crystallinity of a polymer-based on the heat required to melt the polymer.
DSC is a technique which measures heat flow into or out of a polymeric material as a function of time or temperature. So, to calculate the degree of crystallinity of a polymer, specific heat of fusion in J/g is determined from the DSC curve peak area and enthalpy of fusion of 100 % crystalline material is taken from scientific databases. Then, both values are put into the formula given below:
Procedure
1. Take a polymer sample of polyethylene terephthalate of 15 mg weight and clean it and dry it.
2. Place the polyethylene into the sample pan whereas the reference pan is to be kept empty. Both pans are placed on the raised platforms on sensors.
3. Log into the computer and start the DSC 2920 system by pressing the power button on the instrument.
4. A heating rate of 5 ℃/min was used and nitrogen gas is pumped into the system at the rate of 35 ml/min.
5.Now, the sample of polythene is analyzed over the temperature range from 20 ℃ to 180 ℃ then it is cooled from 180 ℃ at 5 ℃/min.
6. After analyzing the sample, DSC software will give peak and curve peak area which will be used to calculate the degree of crystallinity.