Conducting Polymers: Definition, Examples, Properties and Applications
What are Conducting Polymers?
As the name suggests organic polymers that conduct electricity are known as conducting polymers. They are also known as intrinsically conducting polymers (ICPs) and they have alternating single and double bonds along the polymer backbone (conjugated bonds) or that are composed of aromatic rings such as Phenylene, naphthalene, anthracene, pyrrole, and thiophene which are connected through carbon-carbon single bonds.
Examples: Polyacetylene, Polypyrrole, Polyaniline, etc
What is the reason behind conducting nature of these polymers?
Conducting polymers comes in two forms that are doped conducting polymers and non-doped conducting polymers. The conductivity of non-doped conjugated polymers is due to the existence of a conductivity band similar to a metal.
In a conjugated polymer, three of the four valence electrons form strong sigma bonds through sp² hybridization where electrons are strongly localized.
The remaining unpaired electron of each carbon atom remains in a P𝘻 orbital. It overlaps with a neighboring P𝘻 orbital to form a pi bond.
The pi electrons of these conjugated P𝘻 orbitals overlap to form an extended P𝘻 orbital system through which electrons can move free (delocalization of pi electrons). However, the conductivity of non-doped polymers is low.
In the case of doped conjugated polymers, an electron is removed from the valence band by oxidation (p-doping) or is added to the conducting band by reduction (n-doping).
P-doping increases the mobility of electrons in these delocalized orbitals and the polymer becomes highly conductive.
Properties of Conducting Polymers
- Conductivity polymers have high melting and softening points because the mobility of the repeat units is highly restricted due to the presence of a fully aromatic ring structure and the absence of free rotating groups.
- Conductivity polymers show excellent chemical, thermal and oxidative stability due to low hydrogen content and aromatic structure.
- They can be processed into a highly ordered crystalline thin film that is electrically conducting upon doping.
- They are insoluble in many common solvents.
Applications of Conducting Polymers
- They are used in the manufacturing of chemical sensors, electro-magnetic shielding, antistatic coatings, corrosion inhibitors, etc.
- They are also used in compact electronic devices such as polymer-based transitions, light-emitted diode (LEDs), and lasers.
- They are used for microwave-absorbent coating particularly radar-absorptive coatings on stealth aircraft.
- They are used in the manufacturing of printed circuit board because it protects the copper from corrosion and prevents its solderability.
Following are some common examples of conducting polymers:
Examples of Conducting Polymers
1. Polyacetylene
Polyacetylene or Polyethyne having a repeating unit (C₂H₂)ₙ, is a rigid, rod-like polymer that consists of long carbon chains with alternating single and double bonds between the carbon atoms.
It is a conducting polymer whose electrical conductivity was dissolved by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid who received Nobel Prize in chemistry in 2000 for their work.
Structure of Polyacetylene
There are two types of structure of Polyacetylene that is cis- and trans-polyacetylenes.
Preparation of Polyacetylene
Properties of Polyacetylene
- Films of cis-polyacetylene are flexible and can be readily stretched while trans-polyacetylene is much more brittle.
- Both cis and trans-polyacetylene show high thermal stability.
- They are insoluble in common solvents.
Applications of Polyacetylene
- Doped polyacetylene offers a particularly high electrical conductivity therefore it can be used in electric wiring or electrode material in lightweight rechargeable batteries.
- Tri-iodide oxidized polyacetylene can be used as a sensor to measure glucose concentration.
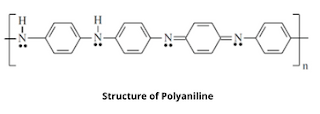
2. Polyaniline
Polyaniline (PANI) is a conducting polymer of the semi-flexible rod polymer family which was discovered in the early 1860s by lightfoot through oxidation of aniline. It behaves like an organic semiconductor that has good electrical conductivity measured in the units s/cm.
Structure of Polyaniline
Preparation of Polyaniline
Polyaniline is synthesized by oxidative polymerization of aniline.
Properties of Polyaniline
- It has great electrical conductivity in the range of 10⁻¹⁰ to 10² s/cm.
- It has band gapes of 4.3 and 2.7 ev in its reduced and oxidized forms respectively.
- It has high chemical stability.
- Polyaniline-based composition can withstand high temperatures like 230-240 ℃ without significant change in electrical properties.
Applications of Polyaniline
- It is used in the manufacturing of printed circuit boards.
- Polyaniline and its derivatives are used as the base element for the production of N-doped carbon materials.
- The color change of polyaniline in different oxidation states can be used in sensors and electrochromic devices.
- Printed emeraldine polyaniline-based sensors have wide application in the electronic sector.
3. Poly-p-phenylene sulphide
Poly-p-phenylene sulphide (PPS) is an organic polymer consisting of aromatic rings linked by sulphides. Though poly-p-phenylene sulphide is insulating in nature, it can be converted into a semiconducting polymer through oxidation or the use of dopants.
PPS is a rigid and opaque polymer with a high melting point (280 ℃). That's why it can be used in filter fabric for coal boilers, film capacitors, and gaskets.
Structure of Poly-p-phenylene sulphide (PPS)
Preparation of Poly-p-Phenylene Sulphide (PPS)
Poly-p-Phenylene Sulphide (PPS) is produced by the reaction of sodium sulphide and dichlorobenzene in a polar solvent such as N-methyl pyrrolidone at high temperature (250 ℃).
Properties of Poly-p-Phenylene Sulphide (PPS)
- It is resistant to heat, acids, alkalies, bleaches, sunlight, and abrasion.
- It absorbs only a small amount of solvent and resists dyeing.
- It has good conducting properties if doped with dopants or by oxidation.
- It has exceptional mechanical strength and good dimensional stability.
Applications of Poly-p-Phenylene Sulphide (PPS)
- It is used in automotive industries for the manufacturing of fuel injection systems, water pump impellers, electric brakes, bulb housing, etc.
- It is used in electronic industries for the manufacturing of connectors, hard disk drives, sockets, switches, etc.
- It is used in the medical industry like surgical instruments, medical fibers, and membranes.
- It is used in fiber extrusion and non-stick and chemical-resistant coatings.
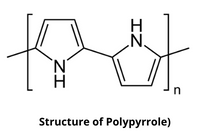
4. Polypyrrole
Polypyrrole (PPy) is an organic polymer having the chemical formula H(C₄H₂NH)ₙH which is obtained by oxidative polymerization of pyrrole. It is an intrinsically conducting polymer that is used in electronics, optical, biological, and medical fields.
Structure of Polypyrrole
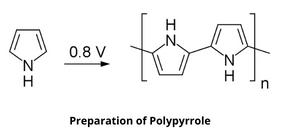
Preparation of Polypyrrole
Polypyrrole can be produced by oxidative polymerization of pyrrole.
Properties of Polypyrrole
- It is an insulator but its oxidized derivatives are good electrical conductors having conductivity in the range of 2 to 1000 s/cm.
- It attains good thermal stability if treated with an acid or base like sulphuric acid and sodium hydroxide.
- It is corrosion resistant and also chemically stable due to cross-linking.
- Its glass transition temperature is 160-170 ℃.
Applications of Polypyrrole
- Polypyrrole and its related polymers are used in electronic devices and chemical sensors.
- It can be used as a potential vehicle for drug delivery.
- It can be used as catalyst support for fuel cells.
- It is used to coat silica and reverse-phase silica to yield a material capable of anion exchange.
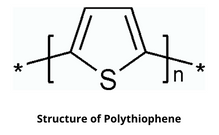
5. Polythiophene
Polythiophene (PT) having the general formula (C₄H₂S)ₙ, is a conductivity polymer whose conductivity exceeds 100 s/cm. The electrical conductivity of polythiophene is due to the delocalization of electrons along the polymer backbone.
The development of polythiophenes was done by Nobel prize winners (2000), Alan J.Heeger, Alan MacDiarmid, and Hideki Shirakawa.
Structure of Polythiophene
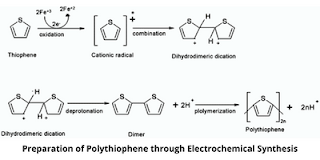
Preparation of Polythiophene
1. Electrochemical Synthesis
Polythiophene can be prepared by electrochemical polymerization of a solution containing thiophene and an electrolyte. Through this method, a conductive polythiophene film gets produced on the anode.
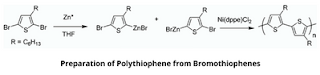
2. From bromothiophenes
2,5-dibromo-3-alkyl thiophene is treated with highly reactive Rieke Zinc to form a mixture of organometallic isomers. Then, the addition of a catalytic amount of Pd (PPh₃)₄ produces a regiorandom polymer, but treatment with Ni(dppe)Cl₂ yields regioregular polythiophene.
Doping of Polythiophene
Polythiophene is an ordinary polymer that becomes electrically conductive upon treatment with an oxidizing agent (electron-acceptors). A variety of reagents have been used to dope polythiophenes like Iodine and Bromine.
Properties of Polythiophene
- It is an excellent intrinsic conducting polymer having conjugated double bonds in the backbone.
- It has high environmental and thermal stability.
- It is a colored solid but tends to be soluble in organic solvents.
- It is transparent having good optical properties.
Applications of Polythiophene
- They are widely used in solar cells due to their ability to form better contact with metal electrodes.
- They are also used in polymer batteries and electrochromic devices.
- They can also work with receptors for detecting metal ions or chiral molecules.
- They show potential in the treatment of prion diseases.



.png)
.png)