U.V-Visible spectroscopy: Instrumentation, Working and Applications
What is U.V-Visible spectroscopy?
U.V-Visible spectroscopy is absorption spectroscopy that deals with the recording of the absorption of electromagnetic radiation of the U.V and Visible regions of the electromagnetic spectrum. The U.V-region ranges from 200-400 nm whereas the visible region ranges from 400 to 800 nm.
So, we can say that U.V-Visible spectroscopy utilizes a 200-800 nm range for working. This technique is widely used for detecting the presence and elucidating the nature of the conjugated multiple bonds and aromatic rings.
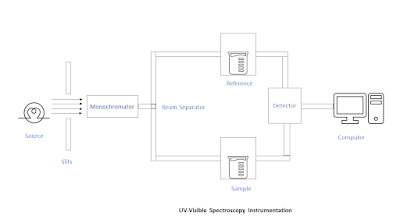
Instrumentation of U.V-Visible spectroscopy
1. Radiation source
Hydrogen-discharge lamp is the most commonly used source of radiation in the U.V region (200-400 nm) whereas a deuterium-discharge lamp is used when more intensity (3-5 times) is desired. A tungsten-filament lamp is used when absorption in the Visible region (400-800 nm) is to be determined.
2. Monochromator
It helps to separate the radiations into separate wavelengths that are it only allow to pass a specific wavelength through it. Monochromators are generally made up of prism or grating which is made up of quartz. This is so because quartz does not absorb the radiations thus ensuring no loss of intensity and precise results.
3. Beam separator
As the name suggests, beam separators help to separate the single radiation into two different paths/chambers that is the reference chamber and the sample chamber. The former is called the reference beam and the latter is known as the sample beam.
4. Detectors
Detectors have photocells or photomultiplier tubes that generate a voltage proportional to the radiation energy that strikes them.
5. Amplifier
The spectrophotometer has a balancing electronic amplifier that subtracts the absorption of the solvent from that of the solution electronically.
6. Recorder
A recorder automatically records the spectrum as a plot of the wavelengths of absorbed radiations against absorbance (A) or molar absorptivity (e).
Working of U.V-Visible spectroscopy
When the U.V-Visible range electromagnetic radiation is emitted by the source, it passes through a monochromator which separates the electromagnetic radiations into separate radiations of different wavelengths.
Then the desired wavelength electromagnetic radiations components are passed through a beam separator which divides the radiations into two chambers that is reference chamber containing reference sample and sample chamber containing actual sample which is to be analyzed.
Then, the radiations penetrate both the samples, and some radiations are absorbed by the sample while some different wavelength is transmitted without any absorbance. These transmitted radiations fall on the amplifier which subtracts the absorption of the solvent from that of the solution. Then finally, the transmitted electromagnetic radiations fall on the detector then recorded by a recorder.
The most important thing is that the U.V-Visible spectrometer does not plot the graph between the transmittance and wavelength instead it plots the graph between absorbance and the wavelength because it is easy for the expert to analyze the graph as it is linear and not inverted.
This is done by taking the log value to transmitted light and incident light as stated by the beers-lambert law that is
Application of U.V-Visible spectroscopy
1. Detection of functional groups
U.V-Visible spectroscopy helps to determine the presence or absence of a functional group in a compound. The absorption of particular wavelength radiation confirms the presence of that group.
2. Detection of the extent of conjugation
U.V-Visible spectroscopy helps to determine the extent of conjugation in compounds, especially in polyenes. This is confirmed by the increase in double bonds, the absorption shift towards the longer wavelength.
3. Identification of unknown compounds
An unknown compound can be identified with the help of U.V-Visible spectroscopy. If the spectrum of an unknown compound is compared with the spectrum of a reference compound and both spectra coincide then it confirms the identification of the unknown compound.