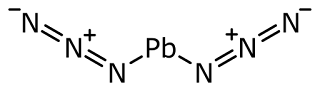Classification of Explosives
What are explosives?
An explosive is a chemical substance that tends to release a great amount of potential energy that can produce an explosion if released suddenly. It is accompanied by the production of light, heat, sound, and pressure.
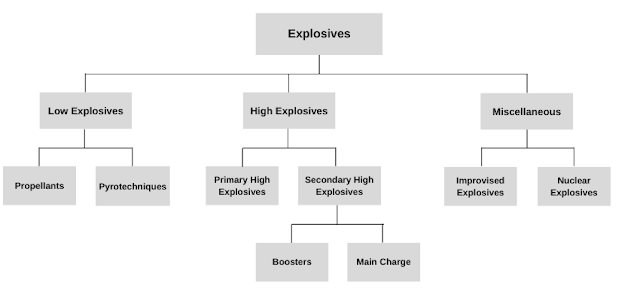
The explosives can be classified based on their chemical composition, velocity of detonation, sensitivity towards heat and light, and physical forms. But broadly explosives are of three types: low explosives, high explosives, and miscellaneous. The latter is subdivided into IEDs and nuclear explosives.
Classification of explosives
1. Low explosives
- Low explosives are chemical compounds in which the rate of decomposition through the material is less than the speed of sound.
- The deflagration rate in low explosives is less than 4000 m/sec.
- The proportion of burning of explosives depends on combustion gas, pressure, grain size, form, and composition.
- Examples of low explosives are gunpowder, flash powder, pyrotechnics, smokeless powder, etc.
(a) Gun powder
- Gunpowder is the first explosive. It is an admixture of sulfur, charcoal, and potassium nitrate.
- The sulfur and charcoal act as fuel while potassium nitrate works as an oxidizer.
- It is widely used as a propellent in firearms and as a pyrotechnic composition in fireworks.
(b) Pyrotechnics
- Pyrotechnics is a process of creating fireworks, safety matches, oxygen candles, explosive bolts, etc.
- This process is based on the fact that these explosives produce exothermic reactions to make heat, light, gas, and sound.
2. High explosives
- High explosives are chemical compounds in which the rate of decomposition through the material is more than the speed of sound (supersonic speed).
- Deflagration rates in high explosives are in between 3 Km/sec to 9 Km/sec.
- They are commonly used for activities like mining, destruction, and military purposes.
- Examples of high explosives are RDX, PETN, TNT, ANFO, etc.
(a) Primary high explosives
- The explosives which are extremely sensitive to mechanical shock, friction, and heat to which they will respond by burning rapidly are known as primary high explosives.
- Examples are mercury fulminate, lead azide, and lead styphnate.
(i) Mercury Fulminate
- It is prepared by dissolving mercury in nitric acid and then pouring it into ethanol.
- It has a molecular weight of 284.6 g and it is insoluble in water.
- It is a colorless crystal.
(ii) Lead azide
- It has a molecular weight of 291.3 g.
- It is insoluble in water.
- It is a colorless crystal.
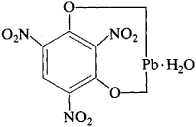
(iii) Lead styphnate
- It has a molecular weight of 468 g.
- It is insoluble in water but sparingly soluble in acetone and ethanol.
- It is of orange-yellow to dark brown color.
(b) Secondary explosives
- They are also known as base explosives.
- They get ignited when exposed to heat or flame in liberated quantities.
- They are added in small amounts in blasting caps to boost their power.
- They are further classified into boosters (RDX, PETN) and main charges (Dynamite, TNT, ANFO).
(i) PETN
- PETN stands for pentaaerythritol tertranitrate
- It is having a molecular weight of 316 g.
- It is a colorless crystal.
- It is soluble in ether and alcohol.
(ii) RDX
- It's IUPAC name is 1,3,5-trinitro-1,3,5-triazinane.
- It is a white solid without any smell or taste.
- It has a molecular weight of 292.1 g.
- It is soluble in acetone.
(iii) TNT
- TNT stands for 2,4,6-trinitrotoluene.
- It is prepared by nitration of toluene with a mixture of nitric and sulphuric acid.
- It is pale yellow.
- It is not soluble in water but soluble in acetone.
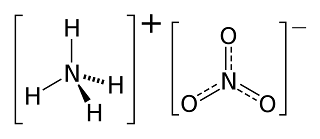
(iv) Ammonium Nitrate
- It has a molecular weight of 80 g.
- It is a crystalline solid with having white or grey color.
- It is sparingly soluble in water.
- It has low shock and friction sensitivities.
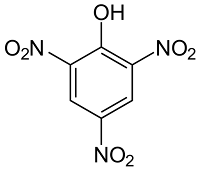
(v) Picric Acid
- It has a molecular weight of 229.1 g.
- It is a colorless to yellow solid crystal.
- It is soluble in ether, alcohol, and acetone.
3. Miscellaneous
(a) Improvised Explosives
- An improvised explosive device (IED) is a homemade bomb to cause destruction.
- It is mainly used by criminals, terrorists, suicide bombers, and insurgents.
- Materials like fertilizers, gunpowder, and hydrogen peroxide can be used as explosives in IEDs.
- IED can be carried or delivered in a vehicle, or packaged and kept roadside.
(b) Nuclear Explosives
- Nuclear explosives contain nuclear active materials like uranium, thorium, etc.
- It is based on a nuclear chain reaction in which neutrons collide with the nuclei of material and initiate a chain reaction.