10 Types of Dyes with their Properties and Dyeing Mechanism
What is a Dye?
A dye is a coloring substance that is used for giving color to different substances or altering the color of something. A dye chemically bonds to the substrate to which it is being applied and has an affinity to adhere to the solvent medium.
A dye contains two groups that are chromophore and auxochrome groups in which a chromophore is a color-bearing group that is responsible for dye color whereas auxochrome is a color helper which is responsible for dye fiber reaction.
Examples of dyes: Indigo dye, Phthalein dyes, Alizarin, etc.
10 Types of Dyes
1. Reactive Dyes
Reactive dyes are those dyes that form a covalent bond between the dye and fiber. These dyes have a reactive group like halo-heterocycle or an activated double bond which once applied to a fiber with a hydroxyl group on the cellulosic fiber.
In a reactive dye, chromophore has a substituent that is activated and allowed to directly react to the surface of the substrate. Some common examples of reactive dyes are Reactive Blue 5, Procion, Primazin, Levafix, etc.
Dyeing mechanism of Reactive Dyes
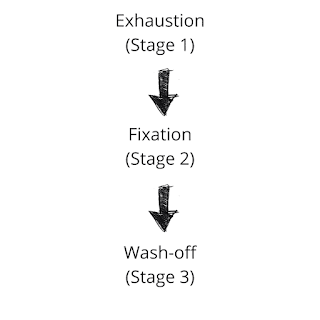
The dyeing of material with reactive dye takes place in three stages:
1. Exhaustion
When the fiber is immersed in a dye liquor, an electrolyte is added to assist the exhaustion of Dye. Generally, NaCl is used as an electrolyte that neutralizes the cotton and helps absorption. So, when the textile material is introduced to dye liquor, the dye is exhausted on the fiber.
2. Fixation
Fixation of dye is the reaction of a reactive group of dye with terminal -OH or -NH₂ group of fiber and thus forming a strong covalent bond with it. This is a crucial phase that is controlled by maintaining proper pH by adding alkali. The alkali is used to create proper pH in the dye bath and works as the dye-fixing agent.
There are mainly two types of reaction that occurs at this stage:
3. Wash-off
After dyeing is completed, a good wash should be applied to the material to remove extra and unfixed dyes from the material surface. This is necessary for levelling and good wash-fastness properties. It is done by a series of hot wash, cold wash, and soap solution wash.
Properties of Reactive dyes.
1. Reactive dyes are found in powder, liquid, and print paste form with all types of shades available.
2. Reactive dyes exhibit good wash and lightfastness with a rating of 5 but have moderate rubbing fastness.
3. Reactive dyes are soluble in water and anionic in nature.
4. Reactive dyes have good perspiration fastness with a rating of 4-5.
Application of Reactive dyes
1. Reactive dyes are used for dyeing cotton or other cellulose fibers at home or in the art studio.
2. Reactive dyes are used for silk painting, screen printing, polychromatic printing, and fabric painting.
2. Acid dyes
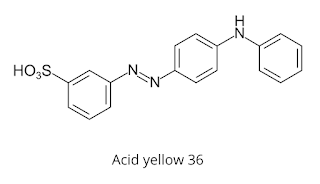
Acid dyes are water-soluble anionic dyes containing sulphonic acid groups (usually present as sodium sulphonate salts). It is applied to fibers like silk, wool, nylon, and modified acrylic fibers using neutral to slightly acidic dye baths. Acid dyes are inexpensive and have a fair light fastness but poor wash fastness.
For example, Acid yellow 36
Dyeing mechanism of Acid dyes
Here we have taken dyeing of wool using an acid dye. So based on the chemical structure of wool, it can be simply represented as H₂N-W-COOH, where 'W' denotes the main non-reacting body of the wool structure. Following are the steps were taken during the dyeing of wool using an acid dye:
1. Submission of wool in water
2. Addition of Acid
3. Addition of Dye
Properties of Acid dyes
1. Acid dyes have substantivity towards protein and polyamide fibers.
2. Wash fastness of acid dye is poor but lightfastness is good.
3. Acid dyes are highly water-soluble and anionic.
4. Acid dyes create ionic bonds with fiber but hydrogen bond and van der Waals forces also contribute.
5. Acid dyes have no affinity for cotton cellulose, hence not suitable for cellulosic fibers.
Application of Acid dyes
1. Acid dyes are used for dyeing animal hair fibers like wool, alpaca, and mohair. They are also effective on silk but least effective on cotton fabrics.
2. Acid dyes are also used in histology to color basic tissue proteins. Lee's stain, Eosin stain are some examples of Acid dyes used in histology.
3. Acid dyes are used for food coloring of cookies, bread, drinks, etc to increase the attractiveness of foods that appeal to more customers. Examples of acid dyes used in food are erythrosine, tartrazine, sunset yellow, and allura red.
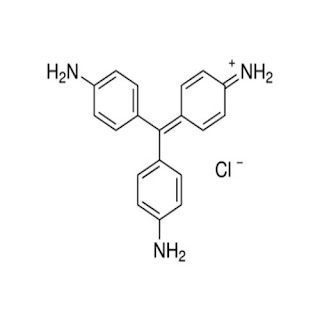
3. Basic Dyes
Basic Dyes are water water-insoluble cationic dyes that contain amino or alkylamino groups having a positive charge which reacts with negatively charged material. Therefore, the primary way of the coloration process with basic dyes is by ionic bonding. Basic Dyes are bright but not very fast to light, washing, and perspiration. They are mainly applied to acrylic fibers.
For example, Basic Brown 1
Dyeing mechanism of Basic Dyes
If we take the case of dyeing of acrylic fiber, it is the anionic property of acrylic fiber which makes it suitable for dyeing with basic dyes (cationic dyes). Since there will be a strong ionic interaction between basic dye and fiber.
Action on acrylic of Basic Dyes
Properties of Basic Dyes
1. Basic Dyes are not soluble in water but soluble in alcohol and methylated spirit.
2. Basic Dyes have poor leveling properties but average fastness properties.
3. Basic Dyes produce very brilliant shades in which the cationic part of the salt is responsible for color production.
4. Basic Dyes have no affinity for cotton but dyeing of cotton can be carried out by mordanting, fixing, and dyeing operation.
Application of Basic Dyes
1. Basic Dyes are used for jute dyeing and jute printing but wool, acrylic fibers can also be dyed using Basic Dyes.
2. Basic Dyes can even color materials like glass, plastic, porcelain, and sealant of sinks.
3. Basic Dyes used in discharge printing, preparing leather, paper, wood, and straw.
4. Basic Dyes can even dye body skin due to negatively charged nucleic acids of cells.
4. Direct Dyes
As the name suggests, Direct dyes can be applied directly to the fabrics from an aqueous solution. They are also known as substantive dyes and are most frequently used as paper dyes.
Direct dyes have sodium salts of Sulphonic acid or carboxylic acid which makes them water-soluble. They also have an azo linkage (-N=N-) which is a leading chromophoric group. The unique property of Direct dyes is that they don't require mordant or binder during dyeing of cotton.
Foe example, Direct Orange 26
Dyeing mechanism of Direct dyes
Direct dyeing is normally carried out in a neutral or slightly alkaline dye bath at boiling point with the addition of either Sodium Chloride (NaCl), Sodium Sulfate (Na₂SO₄), or Sodium Carbonate (Na₂CO₃). The dyeing takes place in three steps that is adsorption, diffusion, and migration.
So, in water, the cellulosic fiber's surface becomes anionic. As the dye is also anionic, neutralizing the surface to remove the repulsion electrolyte is necessary.
Due to swelling in water, the pores of cellulosic fiber's amorphous region get opened and the dyes get diffused inside the pores. Finally, dyes are fixed in the fiber with weak Hydrogen bonds and van der Waals forces of attraction.
Properties of Direct dyes
1. Direct dyes are water-soluble and anionic.
2. Direct dyes are attached to the fiber with weak hydrogen bonds van der Waals forces of attraction.
3. The colors are not brilliant and the fastness properties of direct dyes are also average. For improved fastness, after-treatment is required.
4. The dyeing process requires electrolytes and alkaline solutions.
Application of Direct dyes
1. Direct dyes are used on cotton, paper, leather, wool, silk, and nylon.
2. Direct dyes are also used as pH indicators and as well as biological stains.
3. Direct dyes are used for dyeing dressing gowns and bedspreads which are not washed properly
4. Direct dyes can be applied well at low temperatures and therefore suitable for tie-dyeing and batik work.
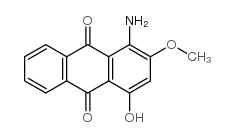
5. Azoic Dyes
The dyes containing at least one insoluble azo group (-N=N-) attached to one or two aromatic rings are called azoic dyes. These dyes are not found in readymade form like other dyes and produced by a reaction between two components that are coupling compound (Naphthol) and Di-azo compound or diazo base or diazo salt.
For example, Bluish red azoic dye
These dyes are used primarily for bright red shades in dyeing and printing because most other classes of fast dyes are lacking in good red dyes.
Dyeing mechanism of Azoic Dye
Following steps are taken during the dyeing of material with Azoic dyes.
1. Naphtholation
In this step, Naphthols which are insoluble in water are converted into the water-soluble compound by treating with alkali.
2. Diazotization
In this step, a base containing an amino group (-NH₂) reacts with the NaNO₂ (Sodium nitrate) to form a solution of diazonium chloride of that base in the presence of excess HCl at 0-5 ℃ temperature.
3. Coupling / Developing
In this final step, the impregnated material is treated in a bath containing diazonium solution to carry out the coupling and thus color is produced inside the fabric.
Properties of Azoic Dyes
1. Azoic dyes are cheap and exhibit excellent washing and lightfastness.
2. Azoic dyes produce intense and bright orange, red and scarlet shades.
3. Azoic dyes have an insoluble azo group present in their structure and hence, they are not water-soluble.
4. The actual color of these dyes depends on diazonium and coupling compounds.
Applications of Azoic dyes
1. Azoic dyes are suitable for cellulose fibers and can be used successfully on protein fibers.
2. Azoic dyes are used widely all over Asia and Australia for batik work.
3. Azoic dyes can be used to give interesting texture color effects on fabric, thread, or paper.
4. Azoic dyes can be used for straight silk painting but it is not advisable due to difficulty in achieving evenness.
6. Disperse Dyes
Disperse dyes are non-ionic, readymade and they are not soluble in water. They are organic coloring substances, suitable for dyeing hydrophobic fibers. Disperse dyes are used for dyeing artificial cellulose ester and synthetic fibers especially acetate and polyester fibers and sometimes nylon and acrylic fibers.
For example, Disperse red 4
Dyeing mechanism of Disperse dyes
The dyeing of material with Disperse dyes takes place in the following simultaneous steps:
1. Diffusion
In the first step, diffusion of dye in the solid phase into water takes place in which breaks up into individual molecules. This diffusion depends on the dispersibility and solubility of dyestuff which is supported by the presence of dispersing agents and increasing temperature.
2. Adsorption
In the second step, adsorption of the dissolved dye from the solution onto the fiber surface takes place. This dyestuff adsorption by fiber surface is influenced by the solubility of the dye in the dye bath.
3. Diffusion of the adsorbed dye
In the final step, adsorbed dye is diffused from the fiber surface into the interior of the fiber substance towards the center. In normal conditions, the adsorption rate is always higher than the diffusion rate.
Properties of Disperse dyes
1. Disperse dyes have low molecular weight, non-ionic, and sparingly soluble in water.
2. Disperse dyes have fair to good light fastness with a rating of about 4-5 whereas wash fastness of these dyes is moderate to good with a rating of about 3-4.
3. Disperse dyes tend to sublime without decomposition due to the absence of ionizable groups. That's why the color of disperse-dyed fabric may fade while ironing.
4. Disperse dyes do not undergo any chemical change during dyeing.
Application of Disperse dyes
1. Disperse dyes are widely used for heat transfer printing (Polysol) in which dye is printed onto paper and heat pressed onto the fabric.
2. Disperse dyes are used to dye cellulose diacetate, cellulose triacetate, and polyester fibers.
3. They can also be applied to nylon and acrylic fibers.
7. Mordant Dyes
Mordant dyes are those dye that requires a binding agent known as mordant for their applications. Mordant acts as a binding agent between the fiber and dye which helps the dye to get attached to the fiber. Mordant dyes are also known as chrome dyes as it contains dichromates and chromium complexes.
Most dyes yield different colors with different mordants and can be used with wool, wool blends, silk, cotton, and certain modified-cellulose fibers.
For example, Mordant red 11
Dyeing mechanism of Mordant dyes
When potassium dichromate (K₂Cr₂O₇) is added to the solution of acid-mordant dyes in the presence of H₂SO₄, a complex is formed with the dyes by chromium ion. The resulting complex is water-insoluble and gets precipitated in the fiber.
Following are the three methods used for mordanting:
1. Pre-mordanting (Chrome)
In this process, the substance is treated with the mordant and then with the dye. The complex between the mordant and dye is formed on the fiber. In this process, the material is impregnated with an insoluble chromium hydrate and then dyeing is done in a separate bath.
2. Meta-mordanting (metachrome)
In this process, dyeing and mordanting are carried out simultaneously in the same bath. The pH of the bath is kept around 6-7 and here the mordant is present in the form of chromate which does not form the lake with the dye and is gradually converted into dichromate.
3. Post-mordanting (After Chrome)
This is one of the oldest and most common mordant dyeing process. Here, the material is first dyed with an acidic dye, and then mordanting with chromium is done in a separate bath or mordanting can also be done in the same bath after exhaustion of the dye has been completed.
Properties of Mordant dyes
1. Mordant dyes exhibit very good light and wash fastness properties with a rating of 4-6.
2. Mordant dyes can combine with metallic oxides to form color lakes
3. Mordant dyes have no affinity for textile fibers instead they attach to fibers with the help of mordants.
4. Mordant dyes have a metal ion (mainly chromium) as a central atom in the structure which is bonded to neighboring -OH, -COOH, or azo group.
Applications of Mordant dyes
1. Mordant dyes are mostly used on natural protein fibers, nylon, and modacrylic fibers.
2. Mordant dyes are also used for the dyeing of cotton and wool.
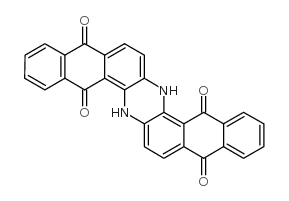
8. Vat Dyes
Vat dyes are the insoluble complex of polycyclic molecules that are based on the quinone structure (keto forms). Vat dyes are synthesised from indigo, anthraquinone and carbazole. The term vat comes from the old indigo method of dyeing in a vat in which reduction of indigo plants takes place through fermentation.
For example, Vat Blue 4
Dyeing mechanism of Vat dyes
The dyeing of Vat dyes takes place in four stages.
1. Vatting
In the first stage, the insoluble dyes are converted into soluble leuco forms by reduction.
2. Diffusion
In this stage, the soluble leuco dyes get inside the cellulosic fiber.
3. Oxidation
In this stage, the soluble dyes are converted into insoluble form again.
4. Washing-off
In the final stage, the unfixed dyes are removed from the surface of the fiber.
Properties of Vat dyes
1. Except for rubbing fastness, Vat dyes exhibit excellent washing, light, and perspiration fastness.
2. Vat dyes are very expensive but the leveling properties are excellent.
2. Vat dyes are found in powder and paste form in which powder form is less stable and paste form is stable in dark places.
4. Vat dyes are moderately soluble in hot water but the solubility can be improved by mixing urea in it at 50-60 ℃ temperature.
Applications of Vat dyes
1. Vat dyes are used in the dyeing of cotton, linen, rayon, wool, silk, and sometimes nylon.
2. Vat dyes are used for dyeing uniforms for armed forces, police, fire, etc which are subjected to severe laundry washing and beaching.
3. Vat dyes are used in outdoor fabrics like parasols, tenting, tarpaulins, etc which require high weather fastness.
4. Vat dyes are used in yarns like sewing threads and color threads for weaving.
9. Sulphur Dyes
Sulphur dyes are usually used to dye cellulosic material and hugely used for producing black and brown shades in cotton. The interaction between the fiber and sulphur dye is established through very strong ionic bonds; which are formed between the anionic groups of the dye and ammonium cations of the fiber.
For example, Sulphur red 7
Moreover, sulphur dyes are water-insoluble dyes and get easily solubilized when it is treated with a weak alkaline solution of sodium sulphide or by any other reducing agent to form leuco compound.
Dyeing mechanism of Sulphur dye
To dye fiber with sulphur dye, the following steps are taken:
1. Dissolving the dyestuff.
2. Reducing the dye to produce a leuco compound.
3. Dyeing with reduced dyes.
4. Washing off the unexhausted dyestuff.
5. Oxidation back to the parent dye.
6. After treatment.
7. Dye fixing treatment.
Properties of Sulphur dyes
1. Sulphur dyes have good leveling and colorfastness properties.
2. Sulphur dyes are cheap and generally produce dark shades like dark greens, dark blues, and blacks.
3. Sulphur dyes are water-insoluble and reducing agents are required to make them soluble.
4. Sulphur dyes are applied in alkali conditions and oxidation is required for color production.
Applications of Sulphur dyes
1. They are used to dye cotton, linen, and rayon fibers.
2. These dyes are used for jigger, winch, and package dyeing of viscose-rayon.
3. They are used to dye khaki color cloths which are used by central armed police forces.