Phenolphthalein: Preparation, Properties and Applications
What is Phenolphthalein?
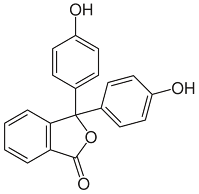
Phenolphthalein is an organic compound having the chemical formula C₂₀H₁₄O₄ that is used as a ㏗ indicator in acid-base titrations. So, as an indicator, it turns pink to red in alkaline and is colorless in acid solutions. In short, it can be written as 'HIn' or '㏗㏗'.
Preparation of Phenolphthalein
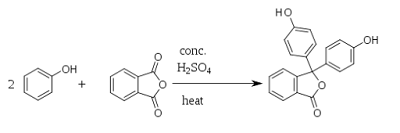
Phenolphthalein can be synthesized by condensation of phthalic anhydride with two equivalents of phenol under acidic conditions.
Properties of Phenolphthalein
- Phenolphthalein is white-yellow, in its crystalline form.
- It is readily soluble in alcohols and mildly soluble in water.
- It does not have taste or smell and it is carcinogenic.
- It appears colorless till pH 8.5 and above that it appears as pink to deep red.
Applications of Phenolphthalein
- It is commonly used as an indicator in acid-base titrations. It turns colorless in acidic solutions and pink in basic solutions.
- It may be used as a laxative but it is not advisable due to the suspected carcinogenicity of this compound.
- It can be used in trap cases during anti-corruption operations as proof of acceptance of bribe. White-colored Phenolphthalein powder spread on currency notes and when any person touches it, the powder gets transferred to his hands. When his hands are washed with a colorless solution of sodium carbonate, it becomes immediately pink confirming the touching of currency notes.
- It is used to check the cement carbonation. If Phenolphthalein is applied to cement undergoing carbonation, it remains colorless (pH 8.5-9) whereas it turns pink when it is applied to normal cement.