Methyl Orange: Introduction, Preparation, Applications
What is Methyl Orange?
Methyl orange is a weak acid that breaks down into orange neutral molecules when it comes into contact with water. Methyl orange shows red color in the acidic medium and yellow color in the basic medium. That's why it is used as a pH indicator in titration due to its clear and distinct color variance properties at different pH.
Preparation of methyl orange
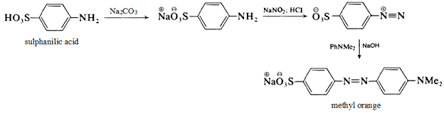
Methyl orange is prepared from sulphanilic acid N, N-dimethylaniline through a diazonium coupling reaction. The first product from the coupling is the bright red form of methyl orange, called helianthin. In the base, helianthin is converted to the orange sodium salt, called methyl orange.
Mechanism of preparation of methyl orange
The mechanism of this reaction is given below:
Step 1:
Sulphanilic acid reacts with sodium carbonate extract and gets converted into sodium salt of p-amino benzene sulphonate
Step 2:
It undergoes a diazotization reaction in presence of nitrous acid to form diazonium chloride salt.
Step 3:
The diazonium chloride then ionizes in an aqueous solution giving sodium ion, chloride ion, and (-)O₃SC₆H₄N₂(+).
Step 4:
N, N-dimethyl aniline hydrochloride added to the (-)O₃SC₆H₄N₂(+) coupling occurs (electrophilic aromatic substitution) to yield methyl orange.
Applications of methyl orange
- It is used as a pH indicator in titration due to its clear and distinct color variance properties at different pH values. That's why methyl orange shows red color in the acidic medium and yellow color in the basic medium.
- It is also used for titrating acids, strong bases, and estimating the alkalinity of water.
- It is employed in the dyeing and printing of textiles.