Atomic Absorption Spectroscopy: Instrumentation, Working and Applications
What is Atomic Absorption Spectroscopy?
Atomic absorption spectroscopy provides us the data about the absorbance of electromagnetic radiation by specific elements. This technique is widely used to determine the presence of unknown elements like alkali and alkali earth metals in the sample.
This is supported by the fact that different metals will absorb different wavelengths of radiation and transmitted radiation is detected by using Lambert Beer's law the graph between the absorbance and the wavelength of the radiation is plotted.
Instrumentation of Atomic Absorption Spectroscopy
1. Radiation source
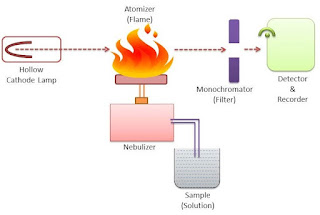
The most common radiation source in Atomic Absorption Spectroscopy is a hollow cathode lamp (HCL). It contains a tungsten anode and a cylindrical hollow cathode made of the element of desire which is the element that is to be determined in the sample.
These anode and cathode are sealed into a glass tube filled with an inert gas like neon or argon. When a voltage is applied between the cathode and the anode then the ionization of the gaseous atom takes place and they start falling on the cathode which is made up of metal.
This initiates the ejection of metal atoms from the cathode which is called sputtering. Some of the sputtered atoms get into an excited state and emit radiation of suitable wavelength when they came back into their original ground state.
2. Sample
The sample is kept in a sample chamber which is connected to a tube with the nebulizer. The sample may be liquid or solid. The solid sample is first dissolved in a solvent and then analyzed. The sample cannot be a gas.
3. Nebulizer
The nebulizer is a closed chamber connected with the sample through a tube. It also has a continuous supply of fuel and oxidants for the smooth flow of the flame. The nebulizer also contains inert gas which acts as a carrier of the sample molecules toward the flame.
4. Burner/Flame
The burner has a continuous glowing flame that is connected to the nebulizer. The flame is continuously glowing through the continuous flow of fuel and oxidants.
It is on the flame in which the atomization of the sample molecule takes place that the atoms of the molecules gas from the lower energy level to the higher energy level and then return to the ground state by emitting the radiation of a suitable wavelength.
5. Lens
The lens is used to converge the emitted radiations into a single path. This is done to avoid the loss of energy from the emitted radiation. This will improve the accuracy of the graph.
6. Monochromator
The monochromator helps to separate the wavelengths of the emitted radiation. A monochromator is adjusted with the help of computer software.
7. Detector
The detector helps to detect the emitted radiation that is passed through the monochromator. A good detector should be able to detect even a weak signal to produce better accuracy.
8. Computer
A computer is an integral part of the flame emission spectrometer. It helps us to draw the graph of the emission spectrum for various atoms of the sample. It is done with the help of suitable software.
Working of Atomic Absorption Spectroscopy
1. Desolvation
The sample is mixed in the solvent in the sample chamber. So, in this step, the metal particles are dehydrated in the flame thus evaporating the solvent. Now only metal particle remains in the flame.
2. Vaporisation
The metal particles are further dehydrated and they get converted into gaseous particles. That's why this process is known as vaporization.
3. Atomization
This is the most crucial step. In this step, the metal ions or molecules get bifurcated into an individual atom with the application of extreme temperature. Different metals have different atomization temperatures, some of them require only less temperature whereas some of them require temperature up to 1000 ℃.
4. Excitation
In this step, the atoms absorb energy from the flame and get transferred from the lower energy state to the higher energy state. The excitation energy will be different for different metals as different elements have different band gaps.
5. Transmittance
The unabsorbed radiation is get transmitted through the atoms of the sample which is received by the monochromator which further separates the radiation as per the desired wavelength which is accepted by the detector and then it is processed in the computer.
It is noted that the value of transmittance is converted into absorbance by Beer-lambert law by taking the logarithm value of the transmittance. This is done for a better interpretation of the resulting graph. Finally, the graph between the absorbance and the wavelength is plotted and the sample is analyzed.
Application of Atomic Absorption Spectroscopy
- It is used for analyzing metals in biological fluids like blood and urine.
- It is used for environmental analysis which is done for monitoring the levels of various elements in the river, seawater, air, etc.
- It is used to monitor the presence of contaminants in food items like fruit juices and wines.
- It is also used for the assay of drugs.